What is Spectroscopy?
Spectroscopy pertains to the dispersion of an object's light into its component colors (i.e. energies). By performing this dissection and analysis of an object's light, astronomers can infer the physical properties of that object (such as temperature, mass, luminosity and composition).
But before we hurtle headlong into the wild and woolly field of spectroscopy, we need to try to answer some seemingly simple questions, such as what is light? And how does it behave? These questions may seem simple to you, but they have presented some of the most difficult conceptual challenges in the long history of physics. It has only been in this century, with the creation of quantum mechanics that we have gained a quantitative understanding of how light and atoms work. You see, the questions we pose are not always easy, but to understand and solve them will unlock a new way of looking at our Universe.
To understand the processes in astronomy that generate light, we must realize first that light acts like a wave. Light has particle-like properties too, so it's actually quite a twisted beast (which is why it took so many years to figure out). But right now, let's just explore light as a wave.
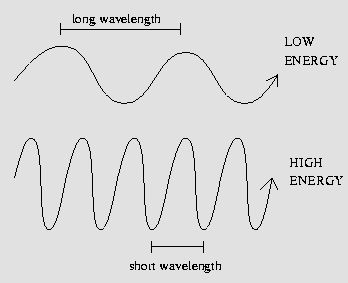
Picture yourself wading around on an ocean beach for a moment, and watch the many water waves sweeping past you. Waves are disturbances, ripples on the water, and they possess a certain height (amplitude), with a certain number of waves rushing past you every minute (the frequency) and all moving at a characteristic speed across the water (the wave speed). Notice the distance between successive waves? That's called the wavelength.

Keeping this analogy in mind, let's leave the ocean beach for a while and think about light like a wave. The wave speed of a light wave is simply the speed of light, and different wavelengths of light manifest themselves as different colors! The energy of a light wave is inversely-proportional to its wavelength; in other words, low-energy waves have long wavelengths, and high-energy light waves have short wavelengths.
Physicists classify light waves by their energies (wavelengths). Labeled in increasing energy, we might draw the entire electromagnetic spectrum as shown in the figure below:
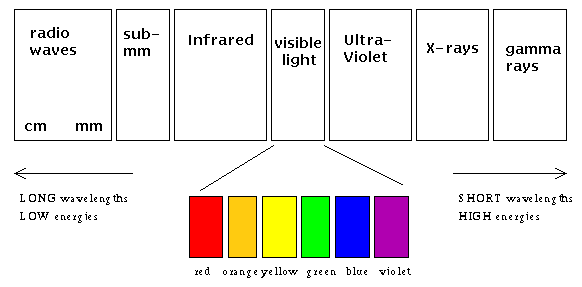
The Electromagnetic Spectrum. Notice how small the visible
region of the spectrum is, compared to the entire range of wavelengths.
Notice that radio, TV, and microwave signals are all light waves, they simply lie at wavelengths (energies) that your eye doesn't respond to. On the other end of the scale, beware the high energy UV, x-ray, and gamma-ray photons! Each one carries a lot of energy compared to their visible- and radio-wave brethren. They're the reasons you should wear sunblock, for example.
When we look at the Universe in a different "light", i.e. at "non-visible" wavelengths, we probe different kinds of physical conditions -- and we can see new kinds of objects! For example, high-energy gamma-ray and X-ray telescopes tend to see the most energetic dynamos in the cosmos, such as active galaxies, the remnants from massive dying stars, accretion of matter around black holes, and so forth. Visible light telescopes best probe light produced by stars. Longer-wavelength telescopes best probe dark, cool, obscured structures in the Universe: dusty star-forming regions, dark cold molecular clouds, the primordial radiation emitted by the formation of the Universe shortly after the Big Bang. Only through studying astronomical objects at many different wavelengths are astronomers able to piece together a coherent, comprehensive picture of how the Universe works!
Typically one can observe two distinctive classes of spectra: continous and discrete. For a continuous spectrum, the light is composed of a wide, continuous range of colors (energies). With discrete spectra, one sees only bright or dark lines at very distinct and sharply-defined colors (energies). As we'll discover shortly, discrete spectra with bright lines are called emission spectra, those with dark lines are termed absorption spectra.
Continuous spectra arise from dense gases or solid objects which radiate their heat away through the production of light. Such objects emit light over a broad range of wavelengths, thus the apparent spectrum seems smooth and continuous. Stars emit light in a predominantly (but not completely!) continuous spectrum. Other examples of such objects are incandescent light bulbs, electric cooking stove burners, flames, cooling fire embers and... you. Yes, you, right this minute, are emitting a continuous spectrum -- but the light waves you're emitting are not visible -- they lie at infrared wavelengths (i.e. lower energies, and longer wavelengths than even red light). If you had infrared-sensitive eyes, you could see people by the continuous radiation they emit!
Discrete spectra are the observable result of the physics of atoms. There are two types of discrete spectra, emission (bright line spectra) and absorption (dark line spectra). Let's try to understand where these two types of discrete spectra.
Emission Line Spectra
Unlike a continuous spectrum source, which can have any energy
it wants (all you have to do is change the temperature), the electron clouds
surrounding the nuclei of atoms can have only very specific energies dictated
by quantum mechanics. Each element on the periodic table has its own set
of possible energy levels, and with few exceptions the levels are distinct
and identifiable.
Atoms will also tend to settle to the lowest energy level (in spectroscopist's
lingo, this is called the ground state). This means that an excited
atom in a higher energy level must `dump' some energy. The way an atom
`dumps' that energy is by emitting a wave of light with that exact
energy.
In the diagram below, a hydrogen atom drops from the 2nd energy level to the 1st, giving off a wave of light with an energy equal to the difference of energy between levels 2 and 1. This energy corresponds to a specific color, or wavelength of light -- and thus we see a bright line at that exact wavelength! ...an emission spectrum is born, as shown below:
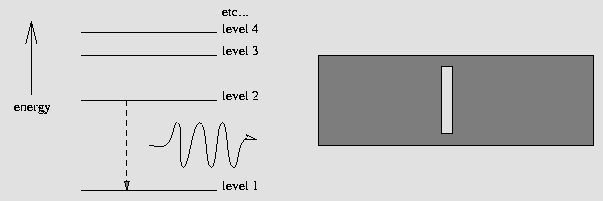
An excited Hydrogen atom relaxes from level 2 to level 1,
yielding a photon. This results in a bright emission line.
Tiny changes of energy in an atom generate photons with small energies and long wavelengths, such as radio waves! Similarly, large changes of energy in an atom will mean that high-energy, short-wavelength photons (UV, x-ray, gamma-rays) are emitted.
Absorption Line Spectra
On the other hand, what would happen if we tried to reverse this process? That is, what would happen if we fired this special photon back into a ground state atom? That's right, the atom could absorb that `specially-energetic' photon and would become excited, jumping from the ground state to a higher energy level. If a star with a `continuous' spectrum is shining upon an atom, the wavelengths corresponding to possible energy transitions within that atom will be absorbed and therefore an observer will not see them. In this way, a dark-line absorption spectrum is born, as shown below:
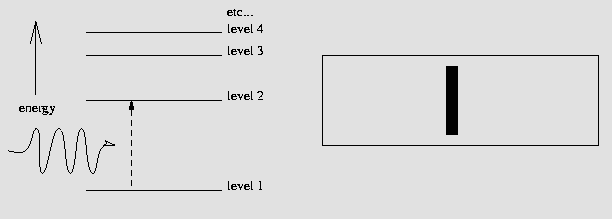
A hydrogen atom in the ground state is excited by a photon
of exactly the `right' energy needed to send it to level 2, absorbing the
photon in the process. This results in a dark absorption line.