
Spectroscopy at Astronomy Camp is done with a spectrograph from Optomechanics Research Inc, coupled with the Mount Lemmon 40" or 60" telescopes and the Camp's SBIG ST6 CCD detector array. With relatively short exposure times, good quality spectra can be taken of most catalogued stars and high surface-brightness deep-sky objects. A few examples of Astronomy Campers' handiwork are shown below.
 |
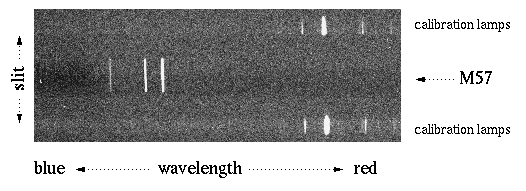
Here's an image of M57 (a.k.a. the Ring Nebula), with a crude representation of the spectrometer slit superimposed. This image is a 180 second exposure using the Camp's ST6 CCD on a 10" Meade Schmidt-Cassagrain telescope. Astronomy Camp's spectrograph was mounted on the Mount Lemmon 60" telescope with the same ST6 CCD. The slit length is about 8 arcminutes long and 1 arcsecond wide; the representation of the slit width in the diagram is exaggerated. |
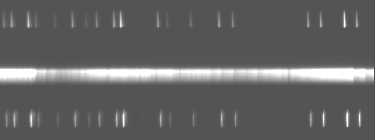
We combined four 5-minute exposures on the Ring Nebula using the 60" and the ST6 camera. We subtracted an appropriate 5 minute dark frame from each image and then combined the images using IRAF. The resulting ST6 image follows: the dispersion (wavelength) axis is horizontal, and the spatial axis (along the Ring Nebula) is vertical. The central lines are M57, the upper and lower spikes are the calibration lamp spectra (Hg+He).
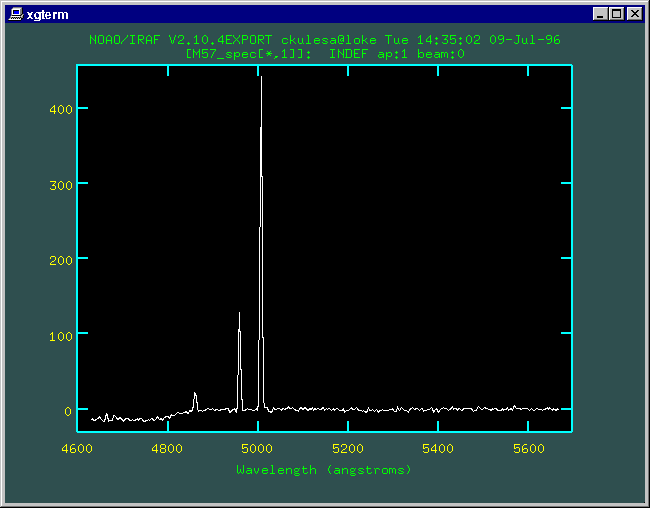
We make a 1-D spectrum from the 2-D image by summing over the aperture of the slit covering M57. Using the well-characterized wavelengths of the calibration lamps, we can use IRAF to register our spectrum to provide a nice wavelength scale. Here's what our spectrum looks like once plotted as intensity versus wavelength.
 |
The emission line at 4861 Angstroms comes from hot, excited atomic hydrogen. Highly-excited hydrogen atoms in M57's gaseous shell, starting in energy level 4, may eventually de-excite to level 2, giving up the energy difference in waves of light at that particular energy (and... they have a wavelength of 4861 Angstroms!). |
The brightest two lines at 4959 and 5007 Angstroms come from twice-ionized oxygen (labeled O++, or O III in spectroscopic notation). This means that two of oxygen's eight electrons have been ripped away. This is a also clue that conditions in this nebula must be harsh. In fact, these lines can only be excited to emit light in temperatures of several thousand Kelvins and rather thin densities of 1-100 atoms per cubic centimeter. There is no continuous spectrum here -- this points out the important fact that planetary nebulae are hot rarified gases -- you see a LINE spectrum. This also points out how astronomers can get valuable information about the physical conditions and important processes in distant astronomical objects.
This spectrum also demonstrates why you can use light-pollution filters (like those made by Lumicon or Orion) to get great contrast from reflection/emission nebulae! These filters pass light waves that lie at wavelengths covered by these three lines, but block light at all other wavelengths. For nebulae, this is very beneficial since they only emit visible light in this wavelength range. You can remove all that ugly skyglow and light pollution without reducing the brighness of the nebula you're looking for.
Would such a narrow-band filter be good for looking at stars or galaxies? Hmm?
Now, onto stellar spectroscopy. This 1/2 second exposure of Sirius is centered near 4000 Angstroms (blue, near-ultraviolet) and clearly shows a series of deep absorption lines. These lines are due to the hydrogen atom. Let's explore how.
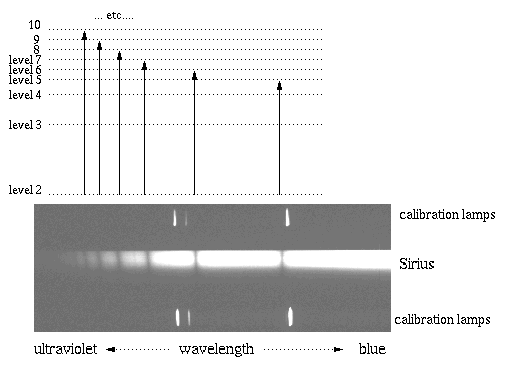
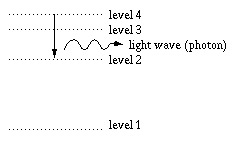
In the cooler outer "atmosphere" around Sirius, mildly excited hydrogen atoms in the 2nd energy level (the 1st excited state) are 'zapped' by photons (light waves) with just the right energy to send them to even higher excited states. In this figure, we match the dark absorption line that results from each transition upward in the hydrogen atom. Notice that the higher-energy transitions on the left result in higher energy absorption lines out in the ultraviolet. This series of lines, starting from level 2, is called the Balmer series after their discoverer.
Stars are classified by their temperatures, which can be determined by the star's spectral features. The hottest stars are termed O-stars, the next cooler are B stars, then A, F, G, K, and finally M-stars. Sirius is a relatively hot A-type star at about 10,000 degrees Kelvin. Such stars have the strongest hydrogen-features (simply due to temperature -- cooler stars can't 'zap' the hydrogen atoms as effectively, and hotter stars will destroy/ionize the hydrogen atoms that create the spectral lines!).
On the other end of the scale -- here is Delta Virgo; a cool M3-type giant star at about 3,500 K and viewed at about 6000 Angstroms (in red light). Note a bright continuum at far left, which suddenly dims into a series of striations (bands). These don't look like the sharp absorption lines of the hydrogen atom, do they? In fact, these bands are due to MOLECULES that can live in the atmospheres of these cool stars! This particular molecule is TiO (titanium oxide). Molecules have a dizzying number of lines because they not only have the electron energy levels like atoms, but also have energy sublevels due to the rotation and vibration of the molecule! At the modest resolution of our spectrometer, these hundreds of lines are blended into absorption bands like what we see here.
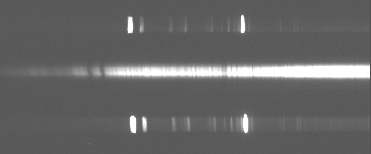
Somewhere between hotter A-type stars and cool M-class stars are stars like our sun, around 5500 degrees Kelvin. Here's Beta-Bootes, a G8 giant star (roughly what our sun will be when it begins dying in about 5 billion years). The first spectrum is at 5500 Angstroms (yellow light), just like the M-star spectrum above. Notice that molecules don't form here (it's too hot for molecules to readily form without being quickly destroyed), but there are still an awful lot of lines around. Most of these features are due to heavy elements -- things like carbon (in several ionization stages), iron, oxygen, magnesium, calcium etc.

This is a spectrum of the same star, but now taken at 4000 Angstroms (deep blue-violet light). The deep absorption lines at left are due to the ion Calcium II (the difference between this and normal, neutral calcium is that one electron has been stripped off here). The small dip in the middle is due to a blend of metallic features and hydrogen. Notice that the hydrogen lines are very weak here -- nothing at all like Sirius (a hotter A-class star).
Craig Kulesa
ckulesa@as.arizona.edu