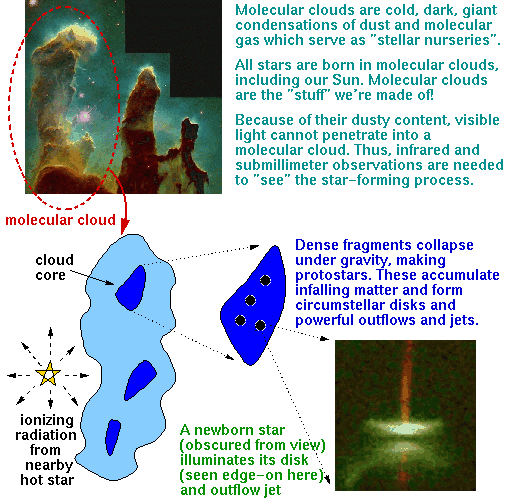
All stars, as far as we know, are born from the gravitational collapse of the core of a molecular cloud.

Molecular clouds are regions of relatively dense interstellar gas and dust that can shield their contents against the destructive ambient ultraviolet (UV) radiation field. In such a cold, protected environment the predominant form of matter, atomic hydrogen, preferentially associates into molecular hydrogen, or H2. However, owing to its simplifying symmetry, cold H2 has no emission line spectrum and therefore remains essentially invisible. However other atomic species, although far less abundant, also associate into common molecules like CO2, H2O, HCN, and so forth, and for the most part glow profusely at microwave radio frequencies and are used as "tracers", or "placeholders" for the otherwise invisible H2 molecule. In fact, over 118 molecules have been detected in dense molecular clouds, some as complicated as the amino acid glycine. This is a stunningly interesting point in regards to the development of life. Consider: if amino acids can form in "empty" space, and amino acids are the building blocks of proteins, and proteins are the building blocks of DNA, and DNA is the building block of life as we know it, is it so far-fetched to imagine the possibility of life elsewhere? Or, stated a different way, it appears that the most fundamental physical processes that serve as necessary conditions for the formation of life on Earth appear to happen elsewhere, and maybe everywhere.
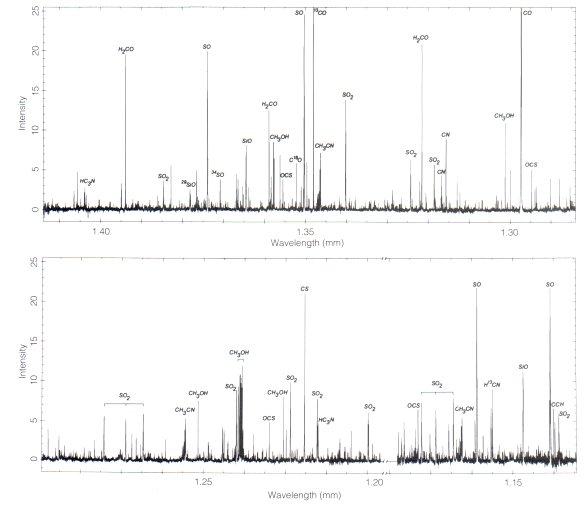
Much data has been gathered on molecular clouds in relation to their ability to form stars, mostly via detection of the molecular emission lines at (sub)millimeter wavelengths. In these clouds, molecules radiate like little microwave radio transmitters as they spontaneously change rotational energy levels. For example, the carbon monoxide molecule (CO) is the most abundant molecule after H2. The first rotational excited state lies only 5 degrees Kelvin (using temperature as a synonym for energy) above the ground state, and therefore is readily excited by the ambient cosmic microwave background radiation or collisions with neighboring molecules (usually H2, since it's 104 times more abundant than even CO). When the CO molecule drops back to the ground state, it gives off a photon of light, in an effort to conserve energy. Because the difference in energy levels is so small, the photon emitted carries away a small amount of energy. For this particular transition in CO, the wavelength of the photon emitted is around 2.6 millimeters, or 115 GHz, in the microwave (radio) portion of the spectrum. This is 1000 times higher in frequency (energy) than what you receive with your FM radio.
Molecules can not only change electronic energy levels like atoms, but also can vibrate and rotate. Each of these new degrees of freedom complicates the spectrum substantially. A spectrum of a nearby star-forming cloud like the Orion Nebula can have thousands of detectable molecular spectral lines even within a narrow range of observed frequency! This is not a nightmare best forgotten, but rather provides a unique diagnostic tool. Each transition of each molecule probes diffeent physical conditions within the cloud -- each spectral line tells a different story, a different perspective. Putting the chorus together remains our best hope for disentangling the complicated physical and chemical structure of molecular clouds, and understanding the initial conditions, or "seeds" of star formation.
In spite of over 30 years of observing molecular clouds using CO as a tracer for the otherwise invisible H2 molecule, there are still many fundamental questions which remain unanswered: